If you produce 36 grams of water and 44 grams of carbon dioxide from 16 grams of methane how many grams of oxygen were needed for the reaction.
The element carbon state of matter at room temperature.
Gases oxygen hydrogen nitrogen and solids carbon phosphorus sulfur and selenium.
Although carbon 14 decays into nitrogen 14 through beta decay the amount of carbon 14 in the environment remains constant because new carbon 14 is always being created in the upper atmosphere by cosmic rays.
While temperature is an easily controlled factor manipulating pressure is another way to cause a phase change.
Other liquid elements.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
The non metals exist in two of the three states of matter at room temperature.
At room temperature it is in a solid state.
Living things tend to ingest materials that contain carbon so the percentage of carbon 14 within living things is the same as the.
An example is the halogen element chlorine.
When pressure is controlled other pure elements may be found at room temperature.
Solid carbon dioxide sublimes everywhere along the line below the triple point e g at the temperature of 78 5 c 194 65 k 109 30 f at atmospheric pressure whereas its melting into liquid co 2 can occur only along the line at pressures and temperatures above the triple point i e 5 2 atm 56 4 c.
The non metals have no metallic luster and do not reflect light.
Its first four.
Carbon is a non metal element.
Carbon is classified as an element in the non metals section which can be located in groups 14 15 and 16 of the periodic table.
Although thermodynamically prone to oxidation carbon resists oxidation more effectively than elements such as iron and copper which are weaker reducing agents at room temperature.
How many grams of carbon monoxide are formed in this reaction.
Gases such as oxygen and solids such as carbon.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
Depending on its form carbon has different.
They have oxidation numbers of 4 3 and 2.
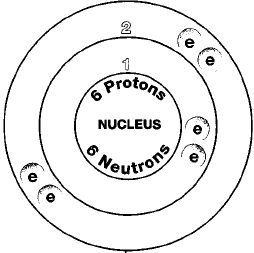
Carbon is the sixth element with a ground state electron configuration of 1s 2 2s 2 2p 2 of which the four outer electrons are valence electrons.
The temperature at which the liquid gas phase change occurs.
Carbon exists in different forms including graphite diamond and graphene.
Non metallic elements exist at room temperature in two of the three states of matter.
That state of matter of an element may be predicted based on its phase diagram.
When methane is burned with oxygen the products are carbon dioxide and water.
Relative atomic mass the mass of an atom relative to that of.